FORM
1 SCIENCE
CHAPTER 1 – INTRODUCTION TO SCIENCE
1.1 What
is Science?
- Science is a set of
knowledge obtained from researches on the natural phenomena which occur on
Earth.
- The examples of
natural phenomena are:
(a) Earthquakes.
(b) Volcanic eruption.
(c) Rainbow formation.
(d) Occurence of rain.
(e) Water evaporation from rivers, seas and pond.
(f)
Solar and lunar
eclipse.
(g) Occurrence of day and night
(h) Occurrence of thunder and lightning.
(i)
A fruit falling
down from the tree.
(j)
Formation of
clouds.
(k) Melting of ice.
(l)
Growth of a child
to adulthood.
(m) A pond appers to be shallower than its actual depth.
- Science can
contribute in the following areas:
|
|
Area |
Importance |
|
(a) |
Industry |
Introduction
of technology to manufacture new products to costumers. |
|
(b) |
Medicine |
Prevention
and treatment of diseases through the application of various new drugs,
sophisticated equipments and technologies. |
|
(c) |
Agriculture |
Invention
of farm machinery, production of eco-friendly pesticides, high quality crops
and the use of modern agricultural technologies. |
|
(d) |
Education |
Makes
teaching and learning process easier and more interesting through various
teaching-aids such as projectors and transparencies. |
|
(e) |
Transportation |
Makes
the movement and travel of man and goods
faster and easier, more efficient and comfortable through various
land, sea and air transports. |
|
(f) |
Information
technology |
Makes
communication and transfer of information (local and international) to be
easier, faster and more efficient through satelittes, internet, handphone and
telefax. |
- The various fields of
science are:
|
|
Fields of science |
Area of study |
|
(a) |
Chemistry |
Matter
and its properties as well as its transformation. |
|
(b) |
Biology |
Living
things. |
|
(c) |
Physics |
Natural
phenomena, mass and energy. |
|
(d) |
Geology |
Rocks
and minerals. |
|
(e) |
Meteorology |
Weather
and climate. |
|
(f) |
Pharmacy |
Medicine |
|
(g) |
Astronomy |
Planets
and stars. |
|
(h) |
Botany |
Plants. |
|
(i) |
Zoology |
Animals |
|
(j) |
Medicine |
Diagnose
and treat diseases. |
- Some careers in
science are:
(a) Doctor
(b) Engineers
(c) Nurses
(d) Botanists
(e) Chemists
(f)
Biologists
(g) Physicists
(h) Geologists
(i)
Pharmacists
(j)
Zoologists
(k) Astronomists
(l)
Astronauts
(m) Meterologists
(n) Nutritionists
(o) Science educators
(p) Computer programmers
1.2 A Science Laboratory
1.
The general rules and safety precautions that must be
observed in a science laboratory are:
(a)
Do not enter a science laboratory without permission from
the teacher.
(b)
Do not play or run around in the science laboratory.
(c)
Do not eat, drink or bring any food and drinks into the
science laboratory.
(d)
Do not play or meddle with any apparatus or chemical in the science
laboratory.
(e)
Do not conduct any experiment without permission from the
teacher.
(f)
Do not taste any chemical unless instructed otherwise by the
teacher.
(g)
Do not smell any gas unless instructed otherwise by the
teacher.
(h)
Do not breathe in any gas too deeply.
(i)
Read the instructions carefully before using any chemical.
Ask the teacher if any doubt about anything.
(j)
Use the apparatus and chemicals carefully and correctly.
(k)
Make sure the workbench is clean and tidy.
(l)
Do not pour any chemical back into its bottle to avoid
contamination.
(m)
Hold the chemical bottles by their labels and not by the
necks.
(n)
Spit out any chemical that happens to be in one’s mouth and
wash it with plenty of water. Then, report to the teacher about it.
(o)
Wash off any chemical that happens to be on one’s skin or
clothing with plenty of water. Then, report to the teacher about it.
(p)
Throw solid waste material into the dustbin. Do not throw it
into the sinkhole.
(q)
Do not direct a test tube with heated substances at oneself
or at anyone else.
(r)
Clean and tidy up one’s place after every experiment.
(s)
Put back all the chemicals and cleaned apparatus back to
their original places after every experiment.
(t)
Do not take out any chemical and apparatus from the science
laboratory.
(u)
Report any accident, injury, breakage or spillage to the
teacher immediately.
(v)
Leave all the doors and windows opened unless instructed
otherwise by the teacher.
(w)
Wash one’s hands after every experiment.
2.
The names and functions of the various apparatus are:
|
|
Apparatus |
Diagram |
Function |
|
|
(a) |
Beaker |
|
To
hold solid or liquid chemicals. |
|
|
(b) |
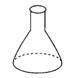
Conical flask |
|
To
hold solid or liquid chemicals. |
|
|
(c) |
Test tubes |
|
To
hold liquid chemicals. |
|
|
(d) |
Filter funnel |
|
To
filter or funnel solid and liquid mixtures |
|
|
(e) |
Crucible |
|
To
hold chemical to be heated |
|
|
(f) |
Cork |
|
As a stopper for test
tube. |
|
|
(g) |
Gas jar |
|
To
collect gas. |
|
|
(h) |
Spatula |
|
To
transfer powder or solid chemicals. |
|
|
(i) |
Evaporating dish |
|
For
evaporating liquid solution. |
|
|
(j) |
Test tube holder |
|
To
hold test tubes during heating. |
|
|
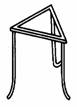
(k) |
Tripod stand |
|
To
support apparatus while heating. |
|
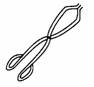
|
(l) |
Crucible tongs |
|
To
hold hot objects or apparatus. |
|
|
(m) |
Glass slide |
|
To
hold specimens for microscopic observation. |
|
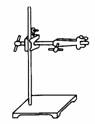
|
(n) |
Retort stand |
|
To
hold apparatus or objects during experiment. |
|
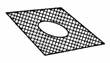
|
(o) |
Wire gauze |
|
To
act as foundation for apparatus during heating. |
|
|
(p) |
Glass rod |
|
For
stirring solutions. |
|
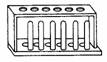
|
(q) |
Test tube rack |
|
To
hold test tubes. |
|
|
(r) |
Bunsen burner |
|
To
provide a fire flame for heating. |
|
|
(s) |
Measuring cylinder |
|
To
measure the volume of a liquid accurately. |
|
|
(t) |
Pipette |
|
To
measure the volume of solution accurately. |
|
|
(u) |
Burette |
|
To
measure the volume of solution accurately. |
|
|
(v) |
Thermometer |
|
To
measure temperature. |
|
|
(w) |
Stop watch |
|
To
measure time. |
|
3.
The proper
methods of using a Bunsen burner are:
(a) Light up the match or lighter before turning on the
Bunsen burner.
(b) Make sure the gas tap and the rubber tube are not
leaking.
(c) Do not put flammable substances near the Bunsen
burner.
(d) Use a water bath when heating flammable substances.
(e) Make sure the gas tap is turned off after every
experiment.
4.
The hazard
symbols their meanings, and examples of chemicals are shown below:
|
|
Symbol |
Meaning |
Examples |
|
(a) |
|
Explosive |
Pottasium,
sodium, hydrogen |
|
(b) |
|
Poisonous |
Mercury,
chlorine, lead, cyanide |
|
(c) |
|
Corrosive |
Concentrated
acid and alkali |
|
(d) |
|
Harmful/ Irritating |
Bromine,
ammonia, chloroform, chlorine |
|
(e) |
|
Flammable |
Alcohol,
yellow phosforus, petrol, kerosene |
|
(f) |
|
Radioactive |
Uranium,
plutonium |
1.3
The steps In A Scientific Investigation
- Scientists can obtain
scientific knowledge through scientific investigation.
- The steps in carrying
out a scientific investigation:
|
Steps |
Scientific investigation |
|
1 |
Identifying
the problem |
|
2 |
Forming
the hypothesis |
|
3 |
Controlling
the variables |
|
4 |
Planning
the experiment |
|
5 |
Carrying
out the experiment |
|
6 |
Collecting
data |
|
7 |
Analysing
and interpreting the data |
|
8 |
Making
conclusion |
|
9 |
Reporting |
- This set of steps in a
scientific investigation can be applied to experiments such as the
experiment to determine the oscillations (complete swings) of a pendulum.
1.4 Physical Quantities And Their Units
- The five physical
quantities and their SI Units are shown below.
|
Physical quantity |
SI Unit |
|
Length |
metre
(m) |
|
Mass |
kilogram
(kg) |
|
Time |
Second
(s) |
|
Temperature |
Kelvin
(K) |
|
Electric
current |
Ampere
(A) |
- The prefixes, their
values and symbols are shown below.
|
Prefix |
Value |
Symbol |
|
Giga |
1
000 000 000 |
G |
|
Mega |
1
000 000 |
M |
|
kilo |
1
000 |
k |
|
deci |
0.01 |
d |
|
centi |
0.01 |
c |
|
mili |
0.001 |
m |
|
micro |
0.000
001 |
m |
|
nano |
0.000
000 001 |
n |
1.5 Concept of Weight and Mass
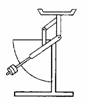
- Weight
(a)
Weight is the force acting on an object towards the centre
of the Earth.
(b)
The SI unit of weight is Newton (N).
(c)
The topics used to measure weight are compression balance
and spring balance.
![]()
spring balance compression balance
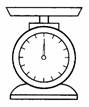
- Mass
(a) Mass is the quantity
(amount) of matter in an object.
(b) The SI unit of mass is
kilogram (kg).
(c) The tools used to measure
mass are, lever balance and beam balance.
lever balance beam balance
1.6 Use of Measuring Tools
1.
Measurement of length.
(a) Units used: milimetre (mm),
centimetre (cm), metre (m) and kilometre (km).
(b) Measuring tools used:
|
|
Diagram |
What to measure |
tool |
|
(i) |
|
Straight
line |
Ruler, measuring tape |
|
(ii) |
|
Curve |
Opisometer,
thread and ruler |
|
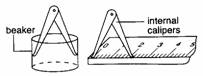
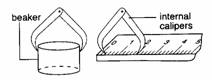
(iii) |
|
Internal
diameter of cylindrical objects. |
Internal
calipers and ruler. |
|
(iv) |
|
External
diameter of cylindrical objects. |
External
calipers and ruler. |
2.
Measurement of area.
(a) Units used: square metre (m2),
square centimetre (cm2)
(b) Measuring tool used:
(i)
Regular shape – graph paper/mathematical formulae.
(ii)
Irregular shape – graph paper .
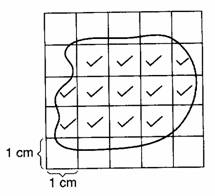
3.
Measurement of volume.
(a)
Units used: cubic metre (m3), cubic centimetre (cm3),
cubic milimetre (mm3), litre
(l)
and mililitre (ml).
(c) Tool used:
(i)
Liquids – measuring cylinder, burette, pipette.
(ii)
Solids – measuring cylinder or eureka can and measuring
cylinder (water displacement method).
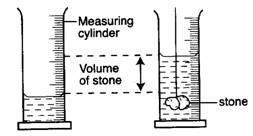
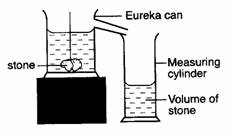
4.
Measurement of temperature.
(a) Unit used: degree Celsius (OC).
(b) Tool used:
(i)
Objects with temperature of between -10 OC to 110
OC – laboratory thermometer.

(ii)
Human body temperature – clinical/doctor’s thermometer.

1.7 The Importance of Using Standard
Units
- The importance of using standard
unit:
(a) facilitating global comunication
various fields.
(b) enabling measurements to be done
accurately.
(c) Enabling data to be analysed,
compared and understood by scientists.